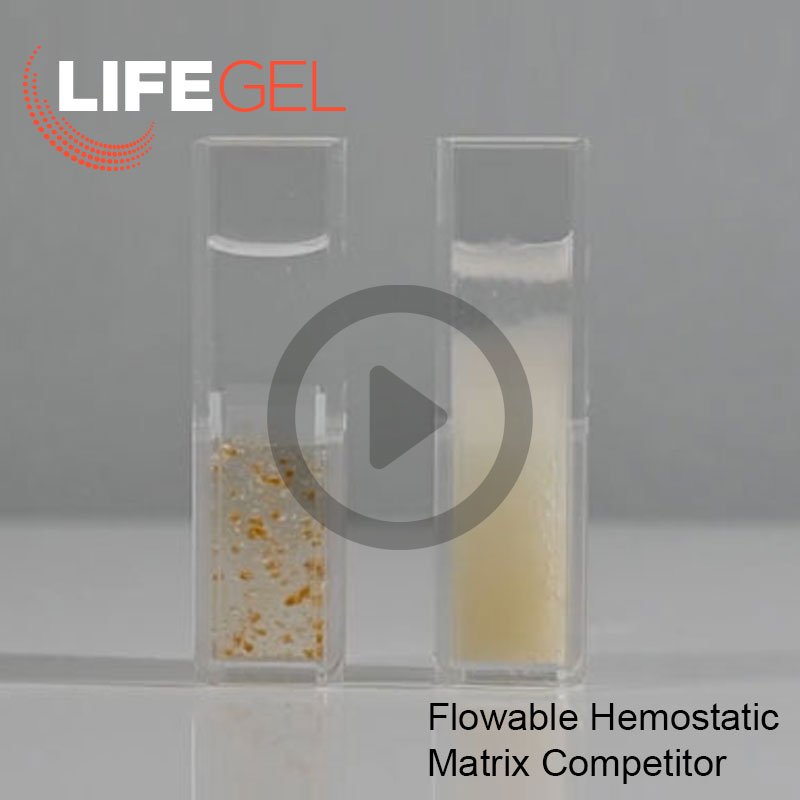
LifeGel™ Absorbable Hemostatic gel
The first and only absorbable hemostatic agent to receive an FDA breakthrough designation.1
.png)
THe First1
On December 20, 2022, Medcura received FDA Breakthrough Device Designation for its LifeGel flowable technology that controls bleeding during surgery, for procedures where swelling cannot be tolerated.
LifeGel is the only absorbable surgical hemostatic technology that has received breakthrough designation from the FDA, for its "no-swell" properties.1 This is the first of its kind in the multi-billion dollar market for surgical hemostatic technologies.
Once approved, LifeGel will be the only absorbable hemostatic agent labeled: “Due to its no-swell properties, LifeGel can be used in surgical procedures where swelling cannot be tolerated.”
The FDA grants this distinctive breakthrough status to expedite the development of devices assessed with the potential to provide more effective treatment for life-threatening, irreversible or debilitating diseases or human conditions (in addition to other criteria).
Current and popular hemostatic flowable technologies can swell.2-9 For complex medical procedures, swelling hemostatic agents can cause nerve injury and even paralysis when used in confined areas.5
See FDA Public Health Notification (Paralysis in Absorbable Hemostatic Agent).
Unlike other flowable hemostatic agents, LifeGel does not swell as it is fully hydrated and ready-to-use.1,2
LifeGel™ for Spine Surgery
Today, surgeons performing spinal procedures have to weigh the risks of using flowable hemostatic agents to control bleeding, against the potential for adverse swelling events. Some popular hemostatic agents may swell after application, potentially causing serious adverse events when applied in bony and confined areas.2-9
The Medcura mission is to accelerate FDA approval for LifeGel™ clinical trials and focus on the spine surgery space, where the risk of swelling in hemostatic agents is the greatest and the market opportunity is the largest.
From there, the path to proving safety and efficacy and the practical and differentiating benefits of being ready-to-use, and easy-to-use, will provide a dynamic runway for initiating a new standard—across the surgical spectrum.
LifeGel aims to address the challenges of current flowable hemostatic agents for spinal procedures. Additional features and benefits for surgical hemostasis include:


No Swelling
Our LifeGel formulation does not swell, potentially mitigating risks associated with swelling hemostatic agents (paralysis, vessel compression, etc.).

Ready to use
LifeGel can be stored at room temperature and requires no mixing or preparation, with great potential to reduce time and waste waste of unused product in the operating room.10

cost-effective
The hemostatic matrix of LifeGel is purposefully designed to be cost-effective and sustainable to produce.*

antimicrobial PROPERTIES
The proprietary base material of LifeGel retains its inherent antimicrobial properties of chitosan, potentially reducing the colonization of bacteria that may lead to infection.11

Relatively transparent
LifeGel's relatively transparent formula allows for greater visibility of the bleeding lesion and clot formation.

mucoadherence
Unlike other flowables, LifeGel both mucoadheres to inverted and vertical tissues.**
The science behind the innovation
Learn more about the development of our proprietary hemostatic platform.

LifeGel™ for Cardiac Surgery
LifeGel™ is uniquely suited for cardiovascular surgery, given its ability to grip and stick to motile, vertical and inverted bleeding sites. The inherent antimicrobial properties of LifeGel™ make it an ideal product to control bleeding during cardiovascular surgery.10
Cardiovascular surgeons face the challenge of surgical site infection pre- and post-cardiac procedures. From sternal edges to pacemaker pockets, cardiovascular surgery sites are prone to mycobacterial colonization and potentially life-threatening complications, that can also severely impede a patient's recovery.


ANTIMICROBIAL PROPERTIES
The proprietary base material of LifeGel retains its inherent antimicrobial properties of chitosan, potentially reducing the colonization of bacteria that may lead to infection.11

READY TO USE
LifeGel can be stored at room temperature and requires no mixing or preparation, with great potential to reduce time and waste of unused product in the operating room.10

COST-EFFECTIVE
The hemostatic matrix of LifeGel is purposefully designed to be cost-effective and sustainable to produce.*

Mucoadherence
Unlike other flowables, LifeGel both mucoadheres to inverted and vertical tissues.**

Relatively TRANSPARENT
LifeGel's relatively transparent formula allows for greater visibility of the bleeding lesion and clot formation.

no swelling
Our LifeGel formulation does not swell, mitigating risks associated with swelling hemostatic agents (potentially mitigating
paralysis, vessel compression, etc.).
The science behind the innovation
Learn more about the development of our proprietary hemostatic platform.

LifeGel™ for Minimally-Invasive Surgery
When working with tiny incisions and camera guidance, any bleeding can quickly obscure the surgical site and compromise the procedure. LifeGel™ is clear when applied, to provide, optimal visibility and ease-of-use of during MIS procedures.
LifeGel is relatively transparent and applies clear, making it easier to use with more visibility of bleed sites and clot progress. Regardless of the angle or orientation of application, LifeGel mucoadheres to achieve hemostasis quickly — and removes easily.


Mucoadherence
Unlike other flowables, LifeGel both mucoadheres to inverted and vertical tissues.**

READY TO USE
LifeGel can be stored at room temperature and requires no mixing or preparation, with great potential to reduce time and waste of unused product in the operating room.10

Relatively TRANSPARENT
LifeGel's relatively transparent formula allows for greater visibility of the bleeding lesion and clot formation.

COST-EFFECTIVE
The hemostatic matrix of LifeGel is purposefully designed to be cost-effective and sustainable to produce.*

DOES NOT SWELL
Our LifeGel formulation does not swell, potentially mitigating risks associated with swelling hemostatic agents (paralysis, vessel compression, etc.).

ANTIMICROBIAL PROPERTIES
The proprietary base material of LifeGel retains its inherent antimicrobial properties of chitosan, potentially reducing the colonization of bacteria that may lead to infection.11
The science behind the innovation
Learn more about the development of the proprietary LifeGel hemostatic platform.

Join Us
Are you interested in learning how to invest? A surgeon interested in participating in a clinical study? A distributor interested in carrying the Medcura portfolio of surgical solutions? Enter your email address below and we'll be in touch.
LifeGel and its application tips are currently in development. All intended uses and/or indications for use for this medical device and its application tips have not been cleared or approved by the FDA.
* Data on file at Medcura. Ongoing exploratory/development testing for safety and performance.
** Data on file at Medcura. Ongoing research for time and cost efficiencies.
- https://www.fdanews.com/articles/210821-medcura-receives-breakthrough-device-designation-for-lifegel-to-manage-surgical-bleeding?v=preview
- Arista ® AH Absorbable Hemostatic Particles, Instructions for Use,; Warnings. Davol Inc. Subsidiary of C. R. Bard, Inc., Warwick, RI; 2015, Arista-AH
- Gelfoam ® absorbable gelatin compressed sponge, USP, Instructions for Use; Warnings. Pfizer, Pharmacia & Upjohn Company LLC, New York, NY; 05/2022
- Gelfoam ® absorbable gelatin powder from absorbable gelatin sponge, USP, Instructions for Use; Warnings. Pfizer, Pharmacia & Upjohn Company LLC, New York, NY; 05/2022,
- PerClot ® Polysaccharide Hemostatic System, Instructions for Use, Warnings. Starch Medical Inc., San Jose, CA, Uploaded 2019, PerClot-IFU
- Surgicel ® Powder, Absorbable Hemostatic Powder, Instructions for Use, Contraindications. Ethicon, LLC, San Lorenzo, Puerto Rico; 05/2017
- Surgicel ® SNoW Absorbable Hemostatic, Instructions for Use, Contraindications; Adverse Events. Ethicon, LLC, San Lorenzo, Puerto Rico; 05/2013
- SurgifloTM Hemostatic Matrix Kit, Essential Product Information, Warnings; Precautions. Ethicon Johnson and Johnson Surgical Technologies; 2022, SURGIFLO-Hemostatic-Matrix-Brochure
- SurgicelTM Original/ Surgicel NU-KNIT/ Surgicel Fibrillar Absorbable Hemostats, Instructions for Use, Contraindications; Adverse Events. Ethicon, Neuchâtel, Switzerland; 06/2013
- Wood, Elizabeth, Advance preparation tends to waste many absorbable hemostatic agents. OR Manager, 03/2017; https://www.ormanager.com/advance-preparation-tends-waste-many-absorbable-hemostatic-agents/
- https://www.mdpi.com/1422-0067/24/13/10540